Lysine-Proline-Valine (KPV)
A tripeptide fragment of α-melanocyte-stimulating hormone (α−MSH) known for its potent anti-inflammatory properties. KPV is researched for its ability to calm inflammation directly at the cellular level, particularly in the context of skin conditions and gut health.
Out of stock
Want to be notified when this product is back in stock?
What is Lysine-Proline-Valine (KPV)?
Overview
Ac-KPV-NH2 (often shortened to KPV in research contexts) is a small, chemically defined tripeptide tool compound based on the C-terminal fragment of α-melanocyte-stimulating hormone (α-MSH 11–13). In published studies, KPV and stabilized analogs like Ac-KPV-NH2 have been used to explore inflammatory signaling, epithelial transport biology, and related downstream ranscriptional responses.
Trusted Peptides supplies this product strictly for in-vitro laboratory research and other controlled research applications.
Key Identifiers
- Short name: Ac-KPV-NH2
- Sequence (core): Lys–Pro–Val (K-P-V)
- Also referenced as: Acetyl-α-MSH (11–13), ACTH(11–13) fragment (context-dependent naming in literature)
- Registry note: “KPV” can refer to the unmodified tripeptide or to capped/amide variants. For identity and lot-specific details (salt form, exact mass, purity), always rely on the accompanying COA.
Common Research Applications
In the peer-reviewed literature, KPV/Ac-KPV-NH2 has been used in preclinical workflows such as:
- Experimental intestinal inflammation models (e.g., cytokine-linked readouts, immune cell infiltration indices, histology scoring endpoints).
- Epithelial transport studies examining transporter-associated uptake (notably PepT1/SLC15A1) and inflammation-linked changes in transporter expression.
- Cell-based pathway assays evaluating inflammatory pathway activity markers, commonly discussed in the context of NF-κB and MAPK signaling under controlled stimulation paradigms.
- Host-defense / antimicrobial assays assessing peptide–microbe interactions in culture systems (results can vary by peptide form and assay conditions).
Mechanistic Context Reported in the Literature
Across preclinical systems, KPV is frequently discussed as a modulator of inflammation-associated signaling and transcriptional control.
In gastrointestinal research, a recurring theme is transporter-associated cellular entry via PepT1 (SLC15A1), which has been reported to be induced in inflamed intestinal epithelium in certain models—providing a framework to study condition-dependent intracellular exposure.
Downstream interpretation in published work typically relies on experimentally measured endpoints (e.g., cytokine-associated measurements, pathway marker assays, leukocyte migration metrics, and tissue histology), rather than on single “headline” outcomes.
Preclinical Research Highlights
Intestinal Inflammation Models
- In mouse colitis models, KPV has been reported to associate with measurable changes in inflammation-related endpoints (e.g., histologic indices, inflammatory marker readouts) compared with controls in the specific study designs.
- Separate work has explored PepT1-mediated uptake and reported inhibition of inflammatory signaling endpoints in cell systems, alongside reduced inflammatory readouts in animal colitis models (study- and protocol-dependent).
- Targeted delivery approaches (e.g., hyaluronic-acid functionalized nanoparticles) have been used in animal studies to investigate localized intestinal exposure and downstream mucosal endpoints.
Inflammation Models Beyond the Gut
The broader melanocortin literature includes evaluation of α-MSH and shorter C-terminal fragments in multiple inflammation paradigms.
When KPV/related tripeptides are studied, discussions often compare receptor engagement and “fragment vs full-length” signaling behavior in the specific assay context.
Antimicrobial Assays (Important Nuance)
Published findings on antimicrobial activity depend heavily on the exact peptide form and the experimental setup.
For example, α-MSH and the KPV tripeptide have been reported to inhibit growth of certain organisms in specific assays, while other studies report limited or no activity for Ac-KPV-NH2 against selected pathogen panels under their conditions.
Form, Quality Control & Documentation
- Form: Typically supplied as a lyophilized (freeze-dried) powder for research workflows.
- Common analytical characterization: Research-grade peptides are often verified using chromatographic purity testing (e.g., HPLC/UPLC) and mass-based identity confirmation (e.g., MS).
- Best practice: Confirm all lot-specific values using the product’s Certificate of Analysis (COA).
Research-Only Notice
For research use only. Not for human consumption.
Scientific references are provided for background only and do not imply endorsement of Trusted Peptides by any authors, institutions, or journals.
There is no affiliation, implied or otherwise, between Trusted Peptides and the researchers cited.
Selected References
- Hiltz ME, Lipton JM. Antiinflammatory activity of a COOH-terminal fragment of the neuropeptide α-MSH (FASEB J, 1989). PubMed
- Richards DB, Lipton JM. Effect of α-MSH(11–13) (lysine-proline-valine) on fever in the rabbit (Peptides, 1984). PubMed
- Kannengiesser K, et al. Melanocortin-derived tripeptide KPV in murine models of inflammatory bowel disease (Inflamm Bowel Dis, 2008). PubMed
- Dalmasso G, et al. PepT1-mediated tripeptide KPV uptake reduces intestinal inflammation (Gastroenterology, 2008). PubMed
- Xiao B, et al. Orally targeted delivery of KPV via hyaluronic acid-functionalized nanoparticles alleviates ulcerative colitis (Molecular Therapy, 2017). PubMed
- Cutuli M, et al. Antimicrobial effects of α-MSH peptides (J Leukoc Biol, 2000). PubMed
- Songok AC, et al. Structural modification studies including Ac-KPV-NH2 and antimicrobial assay outcomes (PLOS ONE, 2018). Full text (PMC)
- Brzoska T, et al. α-MSH and related tripeptides: biochemistry and anti-inflammatory/protective effects (Endocr Rev, 2008). PubMed
Molecular Structure
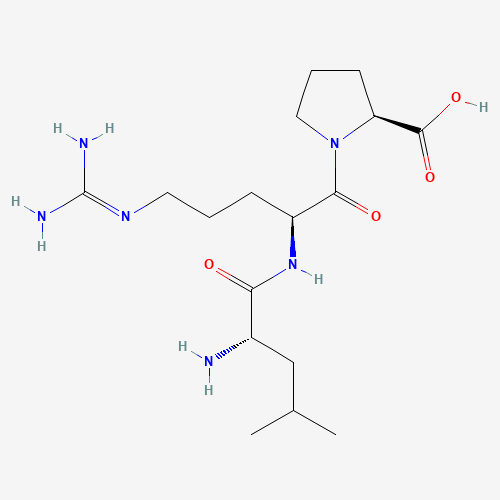
81778-77-6
C17H32N6O4
384.48 g/mol
White to off-white lyophilized powder
Laboratory Tests & Certificates
02172027
Prod. February 4, 2026
Exp. July 14, 2027







